


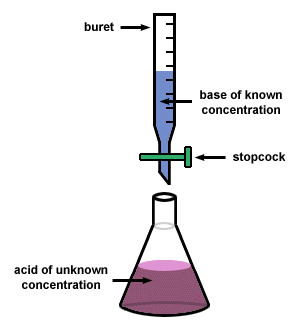
The last two weeks in chemistry lab, we have done titrations! Titrations are always fun. Basically (no pun intended), the students standardize the concentration of a base solution then find the molecular weight of an unknown acid. In order to see when the acid and base has neutralized, we use an indicator called phenolphthalein which is colorless in acidic solution, but pink in basic solutions. To be the most accurate, you want to add enough base to your acid until you have a light pink solution. Of course, you usually get a bright pink solution the first time you do a titration (at least) because you don't know how much base to add. It's usually a competition between the students to see who can get the lightest shade of pink. I of course egg this on by "showcasing" the best solutions. Then the students have bragging rights -which they definitely use. Last week though, I heard one guy exclaim, "Oh man! I added to much! That is the color of failure!"
Apparently, he believed that the fuschia color was failure. Actually, he just knew better next time how much base to add.
1 comment:
That reminds me of Organic Chemistry--can't remember (and don't want to) what we were making, but it took all semester. About half-way through the semester it was supposed to turn from a purple powder to a white one. Mine was purple until the LAST WEEK of the semester! I was SWEATING!
Post a Comment